About NEMESIS
Metabolism disrupting chemicals (MDCs) disturb lipid and glucose metabolism in several metabolically active organs like the liver, pancreas, and the gut, in addition to being able to interfere with many aspects of hormonal action.
Research has found a link between exposure to MDCs with an increased incidence of metabolic diseases such as obesity, steatotic liver disease, and type 2 diabetes. Furthermore, exposure during critical developmental stages can exert life-long, even transgenerational effects. However, the effects of MDCs are still poorly understood and the lack of mechanistic data and predictive models of adverse outcomes hinders their risk assessment and effective regulatory actions.
The “Novel Effect biomarkers for MEtabolic disruptorS: evidence on health Impacts to science and policy needS” (NEMESIS) consortium unites experts in toxicology, medicine, risk assessment, and social sciences to address unmet regulatory needs. We will enhance the understanding on metabolic disruption in the key target organs, the liver, pancreas, and the gut microbiota. By utilizing in silico, in vitro, in vivo, epidemiological, and systems biology approaches, the consortium examines the adverse health effects mediated by MDCs, provide insights from biomonitoring data on MDC levels and MDC mixtures across different regions, increase mechanistic understanding of chemical-induced metabolic disruption, develop predictive computational tools to predict outcomes of exposure, and communicate the project results efficiently to the public to raise awareness and promote health-promoting behaviors.
NEMESIS’ results will improve risk assessment of MDCs in chemical toxicity testing guidelines and facilitate the adoption of alternative models to animal testing. By integrating the project’s findings into regulatory measures, the NEMESIS project aims to enhance the public health of the EU citizens and beyond.
How do we tackle the NEMESIS
Our approach to tackling our NEMESIS is to:
- Identify relevant MDC-mixtures from human biomonitoring data across Europe and use this data to assess the levels of MDC exposure in different regions, assess the effectiveness of chemical policies, identify novel biomarkers for metabolic disruption, and identify relevant mixtures to study further using in vitro and in vivo methods.
- Use state-of-the-art in vitro and in vivo methods to enhance the mechanistic understanding of chemical-induced metabolic disruption and identify novel biomarkers of exposure.
- Improve risk assessment of MDCs.
- Engage citizens in the development of chemical policies and enhance understanding of harmful environmental chemicals and promote adoption of health-promoting behaviors.
Objectives of the project
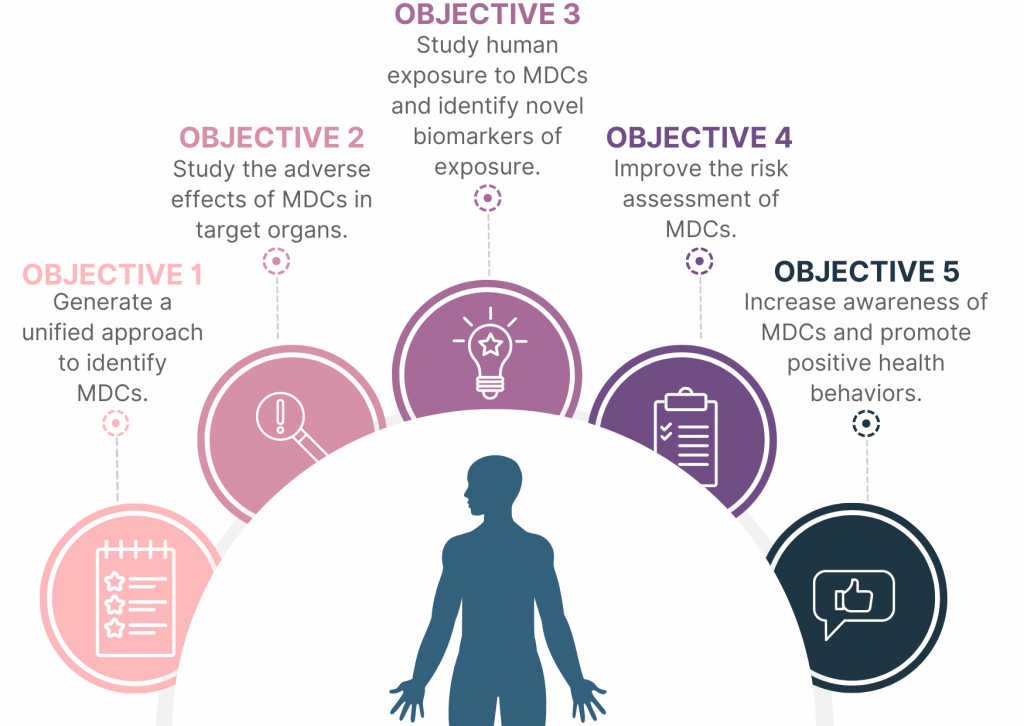
1: Generate a unified approach to identify MDCs.
2: Study the adverse effects of MDCs in targer organs.
3: Study human exposure to MDCs and identify novel biomarkers of exposure.
4: Improve the risk assessment of MDCs.
5: Increase awareness of MDCs and promote health behavior.


